By Anna Davis, UVA ChemSciComm
Article: Jacob E. Walley, Levi S. Warring, Guocang Wang, Diane A. Dickie, Sudip Pan, Gernot Frenking, and Robert J. Gilliard, Jr. Angew. Chem. Int. Ed. 2021, 60, 6682–6690
Catalytic reactions, where small amounts of additives greatly accelerate chemical transformations on a larger scale, are ubiquitous in research and industry. Many common catalysts include transition metals, elements with properties that result in unique catalytic activity. Unfortunately, many transition metals are expensive, toxic, and difficult to dispose, increasing the importance of developing catalysts with other types of elements. Main group metals are one possible alternative, since these could potentially offer similar types of catalytic activity without the associated high costs and safety hazards of transition metals.
The use of bismuth (Bi) in catalysis is relatively underdeveloped because although their corresponding complexes are reactive, they commonly exhibit poor selectivity in a reaction mixture as well as instability in air. If an appropriate fundamental understanding of Bi coordination chemistry can be developed, there is great potential in the field of main group catalysis. The Gilliard group at the University of Virginia has recently isolated a series of novel bismuth-centered complexes to address this knowledge gap.
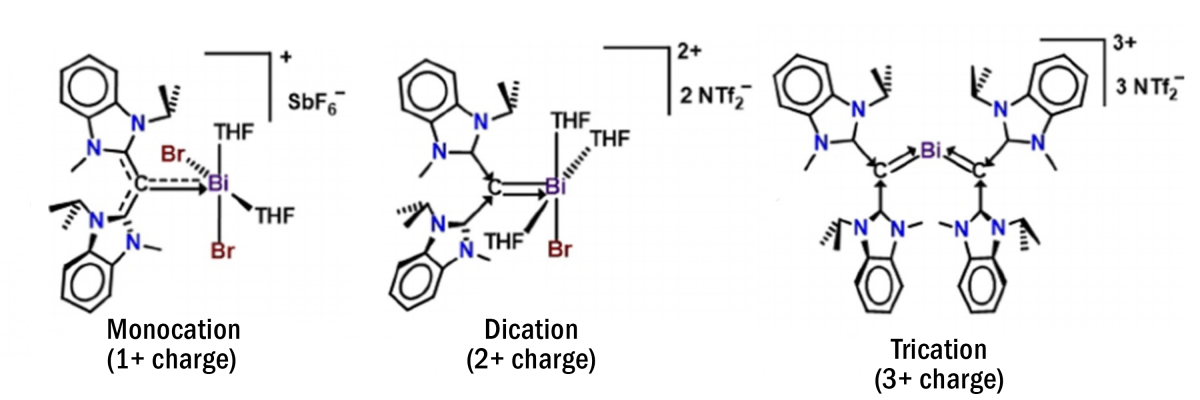
Known bismuth complexes have previously relied on stabilization by nitrogen and chlorine atoms bearing a negative charge, which Gilliard and co-workers were able to exchange for negatively charged carbon atoms. Carbon atoms are generally unstable when bearing a negative charge, but bonding with a bismuth cation (positively charged atom or molecule) benefits both the ligand and the metal center, which represents a significant advancement in the field. This key accomplishment was achieved by carefully designing and changing the groups of atoms which support the negatively charged carbon atoms bound to bismuth, striking a delicate balance between stabilizing the carbon and facilitating bonding. These new structures suggest that additional classes of reactive bismuth complexes are possible which do not rely on nitrogen- and chlorine-stabilized structures.
In addition to creating this new class of molecules, the Gilliard group has developed Bi complexes with overall positive charges ranging from 1+ to 3+. As the complexes become more positively charged, they become more reactive with small molecules such as carbon monoxide, carbon dioxide, and hydrogen gas, which have potential relevance to future catalytic applications. Studies on the ability of these complexes to help harness clean energy are currently underway by the authors.