By: Miranda Wood and Melissa Leyden
UVA ChemSciComm
In recent years, there has been a growing emphasis on wellness and self-care, with individuals looking for new ways to improve their physical and mental health. One area that is often overlooked is hair care, which can have a significant impact on our overall well-being. Understanding the science behind hair and how to properly care for it can lead to stronger hair and a healthier scalp, which can boost our confidence and mood. This article delves into the chemistry of hair care, exploring the composition of hair and common hair-care products, and how it relates to our wellness.
What is hair?
Hair is a protein filament, or a long chain of proteins, that typically grows on most human skin.1 Hair grows in a sac called a follicle made of skin cells in the middle layer of skin, the dermis. While the specific composition of hair is dependent on an individual’s hair type and location on the body, the surface of hair is mostly made of (65-95%) the fibrous protein keratin. The outer layer of a strand of hair is made up of a protective layer of dead cells called the cuticle. The cuticle surrounds the cortex, which is mainly made of lipids and water.(1) The cortex surrounds the medulla, which is the disordered center of the hair shaft that absorbs light and thus determines the color of an individual’s hair.(2)
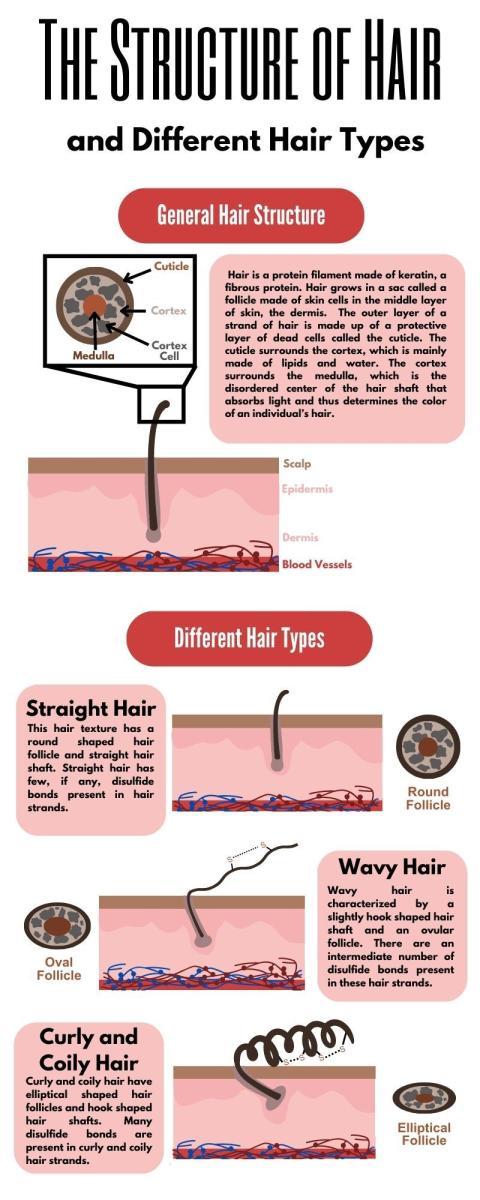
What determines hair texture?
An individual’s natural hair texture is determined by two things: the shape of their hair follicles and the presence or absence of disulfide bonds between cysteine amino acids in the keratin proteins.(3) Curly and coily hair, for example, are the result of hook-shaped hair follicles that allow cysteines to come into close proximity and form more disulfide bonds, creating more texture in the hair. In contrast, straight hair has round follicles, and cysteines are not close enough to form these texturizing disulfide bonds. Therefore, the more hooked the hair follicle and the more disulfide bonds found in the hair, the curlier and coarser it will be.(3) Chemical styling treatments such as relaxers and keratin treatments are the only way to break disulfide bonds. These processes use reducing agents that break disulfide bonds by bonding the participating sulfurs with hydrogens, eliminating the curly texture of the hair.(4)
In addition to disulfide bonds, hair texture is affected by lower energy hydrogen bonds that form between polar amino acids in keratin proteins.(5) Compared to strong disulfide bonds, hydrogen bonds are easily broken through wetting or heat-treating the hair. When water or heat is applied to the hair, the hydrogen bonds break and new ones form as the hair cools or dries, setting the new hairstyle.(6) These hydrogen bonds are also responsible for frizz, which occurs when water in the air breaks the hydrogen bonds, causing the hair to form new hydrogen bonds in an outward position.(6) Adequate moisturizing with conditioners, leave-in moisturizers, and other styling products can prevent this from occurring, thereby eliminating frizziness. The amount of moisture needed depends on an individual’s hair texture, with curly and coily hair requiring more moisturizer as it is more porous and prone to water-loss over time.
What is in my shampoo and conditioner?
Most hair care ingredients are used for cleansing or moisturizing the hair. The most common ingredients in hair cleansing products include sodium lauryl/laureth sulfate, cetyl and stearyl alcohols, and ceramides. These ingredients are primarily used to rid the hair of dirt, oil, products, and environmental build-up. Sodium lauryl/laureth sulfates are surfactants commonly found in drug-store shampoos. These compounds form micelles in water, causing both the foamy texture associated with shampoo and solubilizing oils and dirt in the hair to make them easily rinsable.(7) While sodium lauryl/laureth sulfates are effective cleaning agents, they are often too drying and harsh on curlier and thinner hair types. Cetyl and stearyl alcohols are long-chain fatty acids used as emulsifying agents in many hair cleansers and moisturizers. These fats form a protective, hydrophobic coating on the cuticle of the hair that prevents moisture loss. Finally, ceramides, commonly found in hair-loss prevention and hair strengthening shampoos, is a lipid that forms a waxy layer in the internal hair follicle, helping hair maintain its structure and strength. Together, these and many other ingredients work to create a clean scalp with healthy, nourished hair.(7)

Moisturizing ingredients are a crucial part of hair care products as they help prevent dryness, breakage, and maintain hair vibrance and elasticity. Some of the most common ingredients found in hair conditioners include dimethicone, glyceryl stearate, and cationic conditioning agents. Silicones, most commonly dimethicone, are found in smoothing/frizz-reducing hair care products. Dimethicone, a water-insoluble silicon polymer, coats the cuticle of the hair, making it appear shinier. However, its lack of water solubility results in polymer build-up on the hair and scalp with long-term use, making the hair look weighed down over time. Therefore, products containing these should be used minimally and in moderation.(7) Glyceryl Stearate, on the other hand, is a waxy lipid that aids in solubilizing water and fatty acids, both common ingredients in conditioners. Like cetyl and stearyl alcohols, this compound forms a protective barrier around the hair strand to prevent moisture loss. This oily compound additionally reduces the static electricity of the hair and flatten cuticle scales which reduces hair friction to prevent further damage.(7) Perhaps the most important ingredient in conditioners is a cationic conditioning agent such as behentrimonium chloride (BTAC) and cetrimonium chloride (CETAC). These surfactants carry a positive charge on their head group, which attracts negatively charged amino acids in the keratin proteins in the hair. As a result, the conditioner readily deposits on the hair, particularly in damaged areas where the hair is more negatively charged.(8)
There are a lot of options when it comes to hair care products and routines, which can make decisions about your hair feel overwhelming. However, by understanding the chemistry of hair and their associated products, you can make more informed decisions about the products we use and how they contribute to our overall hair health and wellness.
References
- Content Background: The anatomy and composition of hair – PEP. https://sites.duke.edu/thepepproject/module-2-drug-testing-a-hair-brained-idea/content-background-the-anatomy-and-composition-of-hair/.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4201279/
- Robbins, C. R. Chemical and Physical Behavior of Human Hair; New York Berlin Heidelberg London Paris Tokyo Hong Kong Barcelona Budapest Springer, 1994.
- Wise, L. A.; Palmer, J. R.; Reich, D.; Cozier, Y. C.; Rosenberg, L. Hair Relaxer Use and Risk of Uterine Leiomyomata in African-American Women. American Journal of Epidemiology 2012, 175 (5), 432–440. https://doi.org/10.1093/aje/kwr351.
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M. E. Keratin-Water-NMF Interaction as a Three Layer Model in the Human Stratum Corneum Using in Vivo Confocal Raman Microscopy. Scientific Reports 2017, 7 (1). https://doi.org/10.1038/s41598-017-16202-x.
- Patil, N. V.; Netravali, A. N. Natural “Green” Sugar-Based Treatment for Hair Styling. Fibers 2022, 10 (2), 13. https://doi.org/10.3390/fib10020013.
- Gavazzoni Dias, M. F. Hair Cosmetics: An Overview. International Journal of Trichology 2015, 7 (1), 2. https://doi.org/10.4103/0974-7753.153450
- Rathi, S.; D′Souza, P. Shampoo and Conditioners: What a Dermatologist Should Know? Indian Journal of Dermatology 2015, 60 (3), 248. https://doi.org/10.4103/0019-5154.156355.